- syed.nasar@daneenbio.com
- +91-9321378847
CELL & GENE THERAPY
- By replacing or making mutated cells inactive, cell and gene therapy can hit life-threatening diseases where it hurts the most . In this view, CGT has the potential to emerge as one of the futures biggest catalysts for providing innovative healthcare solutions and improving patient outcomes.
- Biologics and Biosimilars account for the majority of revenue biotherapeutics generated in India .These medicines address the symptoms but not the root cause, leaving a vast opportunity for cell and gene therapy to potentially be integrated into therapeutic strategies to focus on cures instead of symptoms.
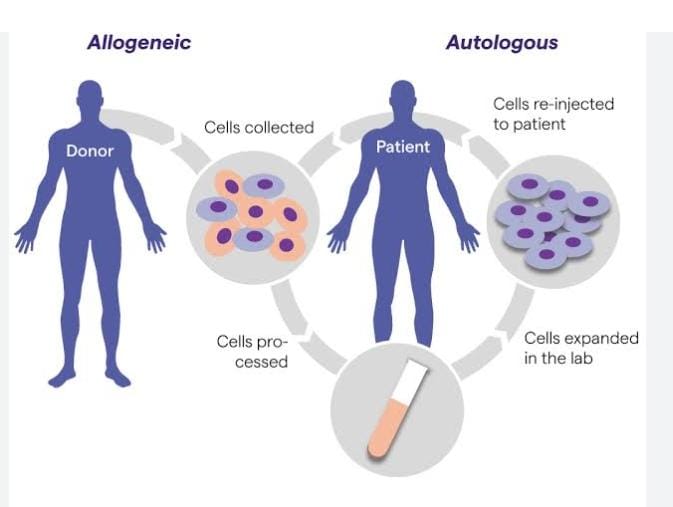
- Traditional medical approaches have, thus far, been symptomatic, but current health challenges call for more modern and new-age treatments. At the helm of these contemporary solutions are cell and gene therapies (CGTs) that modify gene expression in living cells to treat diseases and is already one of the fastest growing fields in healthcare. This is done by using nucleic acids to replace or deactivate a disease-causing gene, or even introduce a new gene into the cells for therapeutic benefit.
- The Indian Council of Medical Research (ICMR) had already issued guidelines in 2019 around procedures to be followed for developing products and conducting clinical trials of gene therapies in India. The Indian government is also launching the ‘Sickle Cell Anaemia Elimination Mission’ that aims to eliminate the disease by 2047. With the government pushing for an increased number of clinical trials, and public institutions like the Indian Institutes of Technology (IITs) joining hands in research and innovation, CGT is creating many opportunities for industry. These could include developing novel therapies, improving manufacturing processes, and conducting clinical trials to generate local data and evidence for specific populations.
- However, the optimism around CGT is not void of challenges and skepticism. The biggest challenge is affordability for patients in India. Cell and gene therapy pricing today is a fraction of costs in Western markets like the US and Europe, but even so, they still pose a challenge in terms of affordability. In addition, the insurance industry is still waking up to CGT as a formidable treatment method. Despite being approved by the Drugs Controller General of India (DCGI), CGT products are yet to find wide coverage by insurance companies. Much of the cost challenges around CGT are due to the high logistics costs of cold chain, wherein CGT samples need to be transported and stored at extreme cold temperatures.
- A way around these challenges could be by integrating CGT with existing treatments, even as subsequent successful clinical trials pave the way for increased reliability. For instance, cancer treatment strategies include surgery, radiotherapy, chemotherapy, or a combination of these methods. However, gene therapy could be combined with other therapeutic approaches and imaging agents to potentially generate more accurate diagnostic and therapeutic strategies. CGT technology and applications are not new. CGT applications are many, from treating leukemia, lymphoma, or myeloma, to T-cell deficiencies, anemia, SCD, and even multiple sclerosis. US and European countries have been conducting clinical trials for over two decades, with the first approved treatments being conducted as early as 2012 in Europe and more recently in 2017 in the US.
- As a demographic giant, India is not far behind. In the last few years, Indian biotechnology and biopharmaceutical companies have launched several CGT products and procedures for oncology, immunocompromised diseases and osteoarthritis. Today, India has at least 10 clinical trials being conducted in gene therapy for diseases such as hemophilia A, B-cell acute lymphoblastic leukemia, type-2 spinal muscular atrophy, and B-cell lymphoma, among others. In fact, in a recent development, biotech startups focusing on CAR-T cell therapies, have attracted healthy funding from well-known pharmaceutical companies. It heralds an era of increased private sector investment in cell and gene therapies.
CELL & GENE THERAPY APPROVED PRODUCT IN INDIA
- IIT-B and Laurus Labs backed ImmunoACT announced the approval of India’s first CAR-T cell therapy, NexCAR19 (Actalycabtagene autoleucel), for the treatment of r/r B-cell lymphomas and leukemia from the Central Drugs Standard Control Organization (CDSCO).
- Immunoadoptive Cell Therapy Private Limited (“ImmunoACT”) received the marketing authorization approval for the first humanized CD19-targeted Chimeric Antigen Receptor T cell (CAR-T cell) therapy product for relapsed/refractory (r/r) B-cell lymphomas and leukemia in India.
- NexCAR19 is an indigenously developed CD19-targeted CAR-T cell therapy. It is the first-of-its-kind, made-in-India product and is a major stride in advanced cell-and-gene therapies.
- Immuno ACT intends to make the NexCAR19 (Actaly cabtagene autoleucel) therapy available to its partner hospitals as soon as possible at 1/10th of Global Cost .
Currently, Indians, who sought CAR-T therapy, had to import the treatment at a substantial cost of Rs 3-4 crore per patient. NexCAR19 is expected to be available at Rs 30-40 lakh per patient.
- At present, they have the capacity to provide personalized therapy to as many as 500 Leukemia and Lymphoma patients annually. They aspire to increase our scale to accommodate 3,000 to 5,000 patients every year for various blood cancers within the next year.
- ImmunoACT aims to expand its CAR-T therapy platform and bring down treatment costs even further, with the goal of reaching Rs 20 lakh per patient. The company has the capacity to treat 500-750 patients per year, and they are looking to further scale up and reduce costs.
- Additionally, they are exploring the potential application of CAR-T therapy for other B-cell-related conditions and researching addressing other types of cancer, such as multiple myeloma and solid tumours
